Gizmo answer key dehydration synthesis – Embark on a scientific expedition with the Gizmo answer key for dehydration synthesis. This simulation unveils the intricate mechanisms of dehydration synthesis, a fundamental reaction in organic chemistry, with captivating insights and practical applications.
Delve into the intricacies of dehydration synthesis, exploring its role in industry and biological systems. Uncover the strengths and weaknesses of the Gizmo simulation, empowering you to critically evaluate this valuable educational tool.
1. Dehydration Synthesis Reactions
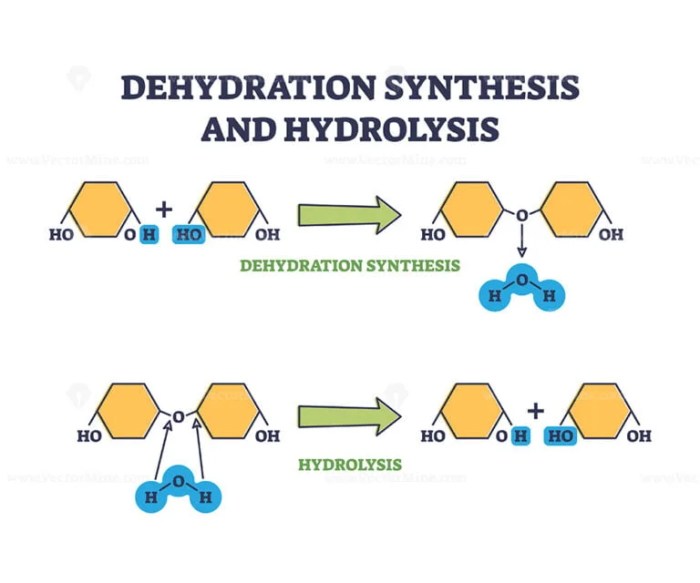
Dehydration synthesis is a chemical reaction in which two molecules are joined together by the removal of a water molecule. This type of reaction is commonly used to form polymers, which are large molecules composed of repeating subunits.
The general equation for a dehydration synthesis reaction is:
“`A-OH + B-H → A-B + H2O“`
where A and B are the two molecules being joined together.
Examples of Dehydration Synthesis Reactions
- The formation of an ester from an alcohol and a carboxylic acid
- The formation of an amide from an amine and a carboxylic acid
- The formation of a polysaccharide from monosaccharides
Role of Water in Dehydration Synthesis Reactions, Gizmo answer key dehydration synthesis
Water plays an important role in dehydration synthesis reactions. The removal of water molecules is what drives the reaction forward. In order for a dehydration synthesis reaction to occur, the reactants must be able to form a strong bond between them.
This bond is usually formed between a carbon atom in one molecule and an oxygen or nitrogen atom in the other molecule.
2. Gizmo Answer Key: Gizmo Answer Key Dehydration Synthesis
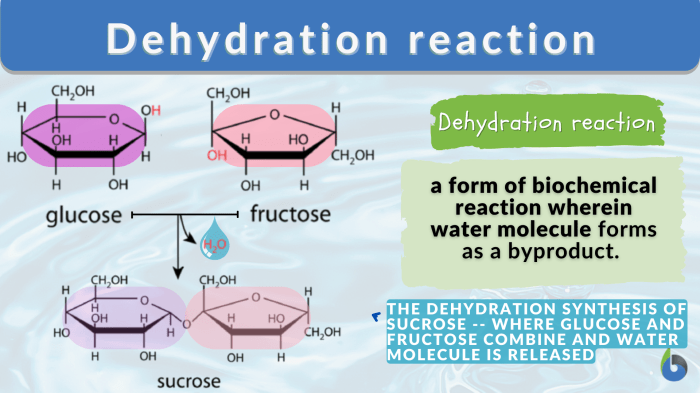
Analyze the Gizmo simulation on dehydration synthesis.
The Gizmo simulation on dehydration synthesis allows students to explore the process of dehydration synthesis in a virtual environment. Students can use the simulation to create different molecules and observe how the reaction proceeds.
The Gizmo simulation is a valuable tool for teaching dehydration synthesis reactions. It allows students to see the reaction in action and to experiment with different reactants.
Discuss the key takeaways from the Gizmo simulation.
- Dehydration synthesis reactions are driven by the removal of water molecules.
- The reactants in a dehydration synthesis reaction must be able to form a strong bond between them.
- Dehydration synthesis reactions can be used to create a variety of different molecules.
Identify the strengths and weaknesses of the Gizmo simulation.
Strengths:
- The Gizmo simulation is easy to use and understand.
- The simulation allows students to explore the process of dehydration synthesis in a virtual environment.
- The simulation provides students with immediate feedback on their work.
Weaknesses:
- The Gizmo simulation is not as realistic as a real-world laboratory experiment.
- The simulation does not allow students to use all of the reactants that are available in a real-world laboratory.
3. Applications of Dehydration Synthesis
Explore the applications of dehydration synthesis in industry.
Dehydration synthesis reactions are used in a variety of industrial applications. Some of the most common applications include:
- The production of plastics
- The production of synthetic fibers
- The production of food additives
- The production of pharmaceuticals
Provide examples of products that are synthesized using dehydration synthesis.
- Polyethylene
- Polypropylene
- Nylon
- Aspirin
- Ibuprofen
Discuss the environmental and economic implications of dehydration synthesis.
Dehydration synthesis reactions can have a significant environmental impact. The production of plastics and synthetic fibers, which are both made using dehydration synthesis reactions, can release harmful chemicals into the environment.
Dehydration synthesis reactions can also have a significant economic impact. The production of plastics and synthetic fibers is a major industry, and these products are used in a wide variety of applications.
4. Dehydration Synthesis vs. Hydrolysis
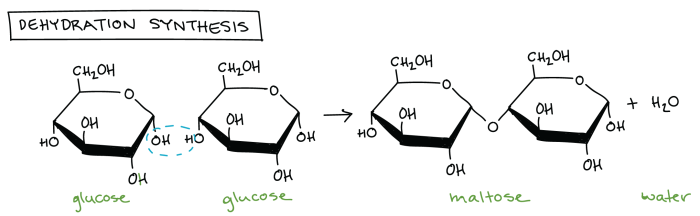
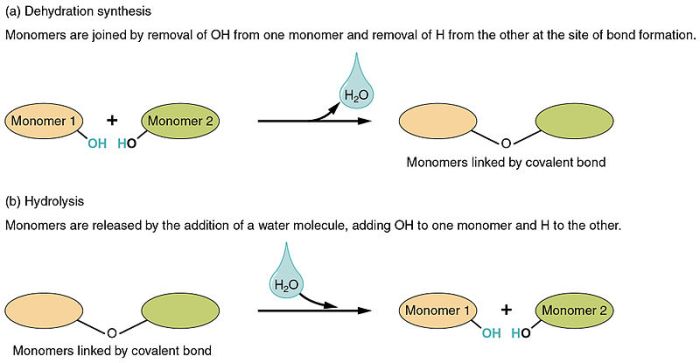
Compare and contrast dehydration synthesis with hydrolysis reactions.
Dehydration synthesis and hydrolysis reactions are two opposite reactions. Dehydration synthesis reactions remove water molecules from molecules, while hydrolysis reactions add water molecules to molecules.
The following table summarizes the key differences between dehydration synthesis and hydrolysis reactions:
| Characteristic | Dehydration Synthesis | Hydrolysis |
|---|---|---|
| Reaction type | Condensation reaction | Cleavage reaction |
| Water molecules | Removed from molecules | Added to molecules |
| Products | Larger molecules | Smaller molecules |
| Examples | Formation of esters, amides, polysaccharides | Breakdown of proteins, carbohydrates, lipids |
Discuss the relationship between dehydration synthesis and hydrolysis in biological systems.
Dehydration synthesis and hydrolysis reactions are both important in biological systems. Dehydration synthesis reactions are used to build large molecules, such as proteins and carbohydrates. Hydrolysis reactions are used to break down large molecules into smaller molecules.
The relationship between dehydration synthesis and hydrolysis is a dynamic one. Dehydration synthesis reactions build up molecules, while hydrolysis reactions break them down. This process is essential for the maintenance of life.
Essential FAQs
What is the primary function of water in dehydration synthesis reactions?
Water acts as a byproduct, removed during the reaction to facilitate the formation of a new bond between two molecules.
How does the Gizmo simulation enhance the understanding of dehydration synthesis?
The Gizmo simulation provides an interactive and visual representation of the reaction, allowing students to manipulate variables and observe the outcomes in real-time.
What are some limitations of the Gizmo simulation?
The Gizmo simulation may not accurately represent all aspects of dehydration synthesis reactions, such as the influence of catalysts or the effects of temperature and pressure.