A gas sample in a rigid container at 455K offers a unique opportunity to explore the intricate relationship between temperature, pressure, and volume in gases. This investigation delves into the fundamental principles of gas behavior, revealing insights into the microscopic world of molecules and their interactions.
The journey begins with an exploration of gas properties and behavior, examining the direct proportionality between temperature and volume at constant pressure. Ideal gases, such as hydrogen and helium, serve as exemplary models for understanding gas behavior. However, the limitations of the ideal gas law are also acknowledged, setting the stage for a deeper understanding of real gases.
Gas Properties and Behavior
Gases are characterized by their low density and ability to expand and fill the available volume. The behavior of gases can be described by the ideal gas law, which relates pressure, volume, temperature, and the number of moles of gas present.
At constant pressure, the volume of a gas is directly proportional to its temperature. This relationship is known as Charles’s law.
Some gases, such as hydrogen, helium, and oxygen, exhibit ideal behavior over a wide range of conditions. However, other gases, such as carbon dioxide and ammonia, deviate from ideal behavior at high pressures and low temperatures.
Limitations of the Ideal Gas Law
- The ideal gas law assumes that gas molecules are point masses with no interactions.
- It does not account for the volume occupied by gas molecules themselves.
- It does not consider the effects of intermolecular forces.
Kinetic Molecular Theory: A Gas Sample In A Rigid Container At 455k
The kinetic molecular theory explains the behavior of gases by assuming that they are composed of tiny, rapidly moving particles that are in constant random motion.
According to the theory, the average kinetic energy of gas molecules is directly proportional to the absolute temperature of the gas.
Assumptions of the Kinetic Molecular Theory
- Gas molecules are point masses with no interactions.
- Gas molecules are in constant random motion.
- The average kinetic energy of gas molecules is directly proportional to the absolute temperature of the gas.
Gas Pressure
Gas pressure is defined as the force exerted by a gas per unit area of a surface.
At constant temperature, the pressure of a gas is inversely proportional to its volume. This relationship is known as Boyle’s law.
Factors that Affect Gas Pressure
- Temperature
- Volume
- Number of moles of gas
Gas Mixtures
A gas mixture is a combination of two or more gases that occupy the same volume.
Dalton’s Law of Partial Pressures
Dalton’s law of partial pressures states that the total pressure of a gas mixture is equal to the sum of the partial pressures of each individual gas.
Gas Laws
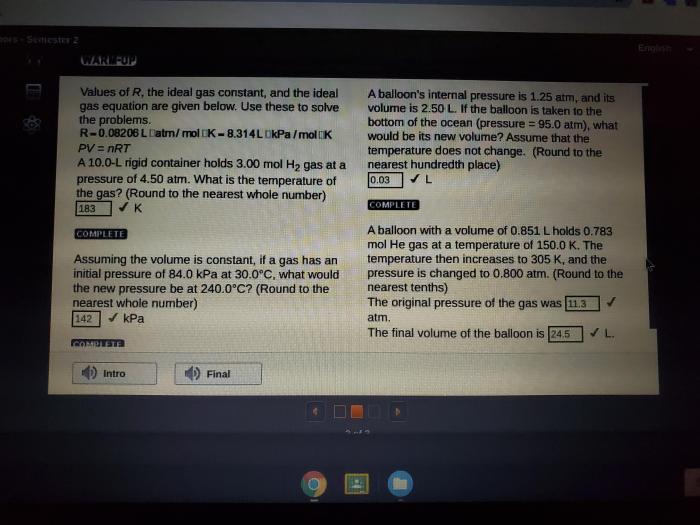
The ideal gas law is a mathematical equation that relates the pressure, volume, temperature, and number of moles of a gas.
Combined Gas Law, A gas sample in a rigid container at 455k
The combined gas law is a combination of Boyle’s law, Charles’s law, and Avogadro’s law.
Gas Phase Reactions
Gas phase reactions are chemical reactions that occur between gases.
Equilibrium in Gas Phase Reactions
Equilibrium in gas phase reactions occurs when the forward and reverse reactions occur at the same rate.
Factors that Affect the Equilibrium Constant
- Temperature
- Pressure
- Concentration of reactants and products
FAQ Insights
What is the relationship between temperature and gas volume at constant pressure?
According to Charles’s Law, the volume of an ideal gas at constant pressure is directly proportional to its absolute temperature.
How does the kinetic molecular theory explain the behavior of gases?
The kinetic molecular theory postulates that gas molecules are in constant random motion and that their average kinetic energy is proportional to the absolute temperature of the gas.
What factors affect gas pressure?
Gas pressure is influenced by temperature, volume, and the number of gas molecules present.