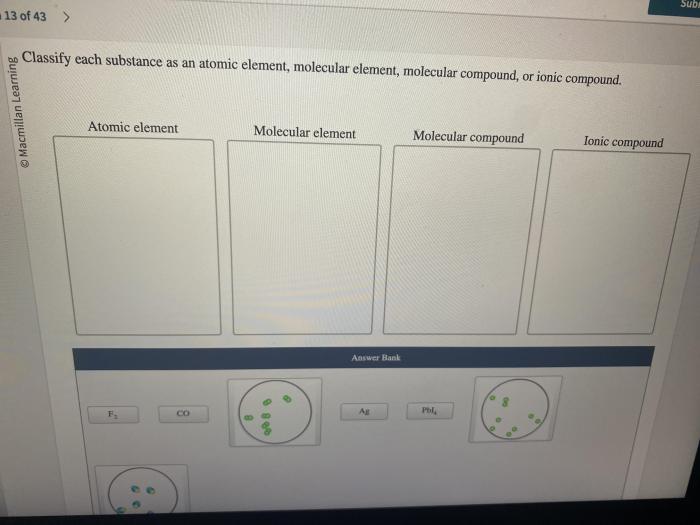
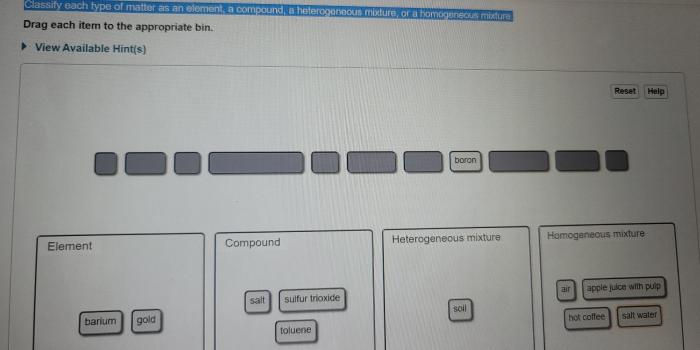
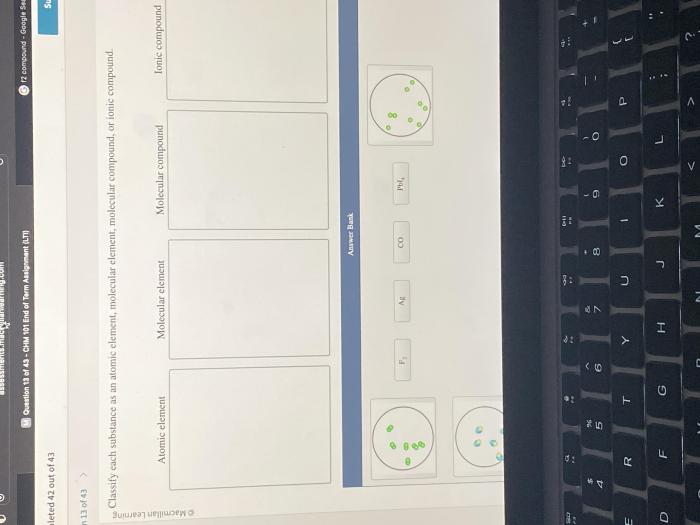
Classify each substance as an element or a compound – Embarking on a scientific exploration, we delve into the realm of classifying substances as elements or compounds. This fundamental distinction forms the cornerstone of chemistry, enabling us to understand the composition and behavior of matter.
Delving deeper, we uncover the unique characteristics of elements, the building blocks of the universe, and contrast them with compounds, intricate combinations of elements. This journey equips us with the tools to identify and classify substances, unraveling their secrets and unlocking their potential.
Defining Elements and Compounds
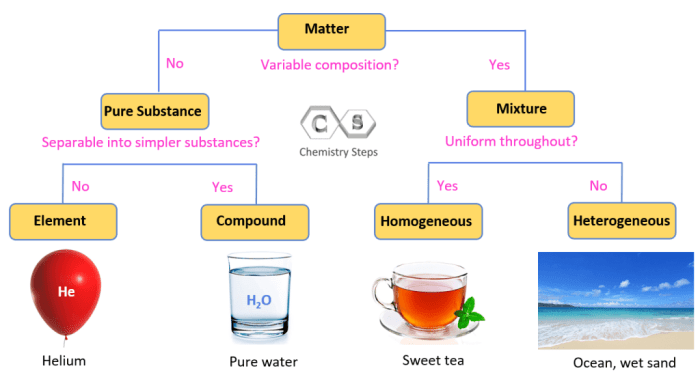
An element is a pure substance that cannot be broken down into simpler substances by chemical means. It consists of only one type of atom, which is the basic unit of matter. Elements are the building blocks of all matter and are represented by chemical symbols, such as H for hydrogen, O for oxygen, and Fe for iron.
A compound, on the other hand, is a substance composed of two or more different elements chemically combined in fixed proportions. Compounds can be broken down into their constituent elements by chemical reactions. Unlike elements, compounds have unique chemical formulas that indicate the ratio of the elements present.
For example, water (H2O) is a compound composed of two hydrogen atoms and one oxygen atom.
Identifying Elements and Compounds
Elements can be identified by their unique chemical symbols, which represent the first one or two letters of the element’s name. Compounds, on the other hand, are identified by their chemical formulas, which indicate the elements present and their relative proportions.
Elements typically have simple chemical symbols, such as H for hydrogen or Fe for iron. Compounds, however, have more complex chemical formulas that reflect the ratio of the elements present. For example, water has the chemical formula H2O, indicating that it is composed of two hydrogen atoms and one oxygen atom.
Classifying Substances
| Substance | Symbol/Formula | Element/Compound | Reasoning |
|---|---|---|---|
| Hydrogen | H | Element | Consists of only one type of atom (hydrogen). |
| Water | H2O | Compound | Composed of two different elements (hydrogen and oxygen) in fixed proportions. |
| Sodium chloride | NaCl | Compound | Composed of two different elements (sodium and chlorine) in fixed proportions. |
| Gold | Au | Element | Consists of only one type of atom (gold). |
| Carbon dioxide | CO2 | Compound | Composed of two different elements (carbon and oxygen) in fixed proportions. |
Examples and Non-Examples
Some examples of elements include hydrogen (H), oxygen (O), iron (Fe), and gold (Au). Examples of compounds include water (H2O), sodium chloride (NaCl), and carbon dioxide (CO2).
Substances that may initially be confusing to classify include diatomic molecules and ionic compounds. Diatomic molecules, such as H2 and O2, are composed of two atoms of the same element and are considered elements. Ionic compounds, such as NaCl and KCl, are composed of positively charged ions (cations) and negatively charged ions (anions) and are considered compounds.
Applications of Classification, Classify each substance as an element or a compound
Classifying substances as elements or compounds is essential in various fields, including chemistry, materials science, and environmental science. It helps us understand the structure, properties, and reactivity of substances, as well as how they interact with each other.
For example, in chemistry, classifying substances as elements or compounds allows us to predict their chemical behavior and reactivity. In materials science, it helps us design and develop new materials with specific properties. In environmental science, it enables us to understand the fate and transport of pollutants in the environment.
General Inquiries: Classify Each Substance As An Element Or A Compound
What is the key difference between an element and a compound?
An element is a pure substance composed of only one type of atom, while a compound is a substance composed of two or more different types of atoms chemically bonded together.
How do we determine whether a substance is an element or a compound?
We can determine the elemental or compound nature of a substance by examining its chemical composition. Elements consist of a single type of atom, whereas compounds contain multiple types of atoms.
What is the significance of classifying substances as elements or compounds?
Classifying substances as elements or compounds is crucial for understanding their chemical properties and behavior. It allows us to predict their reactivity, stability, and potential applications.