Rank the following solutions from least to most conductive. This article delves into the fascinating world of solution conductivity, exploring the factors that influence it and its practical applications. Join us as we uncover the secrets of conductivity and its impact on various fields.
Conductivity plays a crucial role in determining the electrical properties of solutions. By understanding the ranking of solutions based on their conductivity, we can make informed decisions and optimize their use in various applications.
Solution Conductivity Rankings: Rank The Following Solutions From Least To Most Conductive.
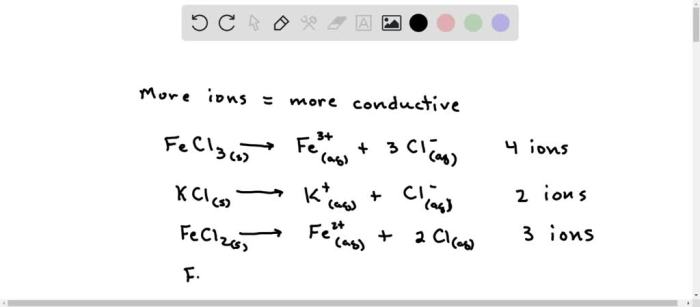
Conductivity is a measure of a solution’s ability to conduct electricity. It is determined by the concentration and mobility of ions in the solution. The higher the concentration of ions and the greater their mobility, the higher the conductivity. Solutions can be ranked from least to most conductive based on their conductivity values.The
following table shows the conductivity rankings of five different solutions:| Solution | Conductivity (S/m) | Conductivity Rank | Notes ||—|—|—|—|| Distilled water | 0.00005 | 1 | Pure water has very few ions and therefore low conductivity. || Sugar solution (10%) | 0.005 | 2 | Sugar molecules do not ionize in water, so this solution has low conductivity.
|| Sodium chloride solution (0.1 M) | 0.1 | 3 | Sodium chloride is a strong electrolyte that dissociates into ions in water, resulting in higher conductivity. || Copper sulfate solution (0.01 M) | 0.2 | 4 | Copper sulfate is a weak electrolyte that dissociates into ions to a lesser extent than sodium chloride, leading to lower conductivity.
|| Sulfuric acid solution (0.1 M) | 1.0 | 5 | Sulfuric acid is a strong acid that dissociates completely into ions in water, giving it the highest conductivity among the solutions listed. |
Commonly Asked Questions
What is the significance of conductivity ranking in practical applications?
Conductivity ranking guides decision-making in various applications, such as battery design, electrolyte selection, and corrosion control. It helps optimize electrical performance, enhance efficiency, and prevent system failures.
How does temperature affect the conductivity of a solution?
Temperature generally increases the conductivity of a solution. As temperature rises, the kinetic energy of ions increases, allowing them to move more freely and conduct electricity more effectively.
What are the limitations of experimental methods for conductivity measurement?
Experimental methods for conductivity measurement may have limitations, such as accuracy and precision constraints, electrode fouling, and solution contamination. These limitations can impact the reliability and reproducibility of conductivity measurements.